Sbírka 95 Atom Parts And Description Vynikající
Sbírka 95 Atom Parts And Description Vynikající. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a.
Nejchladnější Questions And Answers How Do I Make A Model Of An Atom
Each of these parts has an associated charge, with protons carrying a. Learn more about thomson's model of atom in detail. According to his atom diagram, the atom has a small, positively charged nucleus in center. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.
The origin of the word atom comes from the greek word, which means indivisible. Each of these parts has an associated charge, with protons carrying a. The structure of atom consists of two parts: The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. An atom is composed of two regions:

The origin of the word atom comes from the greek word, which means indivisible. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

Now let us study the structure of atom to further understand how the atoms react, behave and interact... This nucleus carries the entire mass of the atom. As such, the atom is the basic building block of chemistry. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. At the time these particles were baptized it was believed that they could not actually be divided. The structure of atom consists of two parts: Each of these parts has an associated charge, with protons carrying a. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom.

According to his atom diagram, the atom has a small, positively charged nucleus in center. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Each of these parts has an associated charge, with protons carrying a. The origin of the word atom comes from the greek word, which means indivisible. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. This nucleus carries the entire mass of the atom. According to his atom diagram, the atom has a small, positively charged nucleus in center. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom is composed of two regions: The structure of atom consists of two parts: Each of these parts has an associated charge, with protons carrying a.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the... This nucleus carries the entire mass of the atom. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.

According to his atom diagram, the atom has a small, positively charged nucleus in center. Learn more about thomson's model of atom in detail. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a.. As such, the atom is the basic building block of chemistry.

The origin of the word atom comes from the greek word, which means indivisible. Each of these parts has an associated charge, with protons carrying a. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Learn more about thomson's model of atom in detail. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. As such, the atom is the basic building block of chemistry. The origin of the word atom comes from the greek word, which means indivisible. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact... Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.

Each of these parts has an associated charge, with protons carrying a. An atom is composed of two regions: Learn more about thomson's model of atom in detail. The structure of atom consists of two parts:. Each of these parts has an associated charge, with protons carrying a.

Learn more about thomson's model of atom in detail. At the time these particles were baptized it was believed that they could not actually be divided. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. This nucleus carries the entire mass of the atom. Learn more about thomson's model of atom in detail. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Each of these parts has an associated charge, with protons carrying a. According to his atom diagram, the atom has a small, positively charged nucleus in center.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.

According to his atom diagram, the atom has a small, positively charged nucleus in center... According to his atom diagram, the atom has a small, positively charged nucleus in center. Each of these parts has an associated charge, with protons carrying a.

Learn more about thomson's model of atom in detail... According to his atom diagram, the atom has a small, positively charged nucleus in center. Now let us study the structure of atom to further understand how the atoms react, behave and interact. The origin of the word atom comes from the greek word, which means indivisible. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. As such, the atom is the basic building block of chemistry. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Learn more about thomson's model of atom in detail. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Each of these parts has an associated charge, with protons carrying a.. This nucleus carries the entire mass of the atom.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the... According to his atom diagram, the atom has a small, positively charged nucleus in center. Each of these parts has an associated charge, with protons carrying a. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Learn more about thomson's model of atom in detail. As such, the atom is the basic building block of chemistry... Now let us study the structure of atom to further understand how the atoms react, behave and interact.
The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Learn more about thomson's model of atom in detail. According to his atom diagram, the atom has a small, positively charged nucleus in center. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. An atom is composed of two regions:. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

According to his atom diagram, the atom has a small, positively charged nucleus in center... The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. An atom is composed of two regions:

The structure of atom consists of two parts:. Each of these parts has an associated charge, with protons carrying a. An atom is composed of two regions: It also is the smallest unit of matter that has the characteristic properties of a chemical element... This nucleus carries the entire mass of the atom.

At the time these particles were baptized it was believed that they could not actually be divided.. This nucleus carries the entire mass of the atom. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. At the time these particles were baptized it was believed that they could not actually be divided.. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Learn more about thomson's model of atom in detail. An atom is composed of two regions:.. At the time these particles were baptized it was believed that they could not actually be divided.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Learn more about thomson's model of atom in detail. At the time these particles were baptized it was believed that they could not actually be divided. This nucleus carries the entire mass of the atom. The structure of atom consists of two parts: Each of these parts has an associated charge, with protons carrying a. The origin of the word atom comes from the greek word, which means indivisible. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. As such, the atom is the basic building block of chemistry. An atom is composed of two regions: An atom is composed of two regions:

Each of these parts has an associated charge, with protons carrying a. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. According to his atom diagram, the atom has a small, positively charged nucleus in center.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.. According to his atom diagram, the atom has a small, positively charged nucleus in center. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. An atom is composed of two regions:

Each of these parts has an associated charge, with protons carrying a. The structure of atom consists of two parts: The origin of the word atom comes from the greek word, which means indivisible. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.. Each of these parts has an associated charge, with protons carrying a.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. The structure of atom consists of two parts:. As such, the atom is the basic building block of chemistry.
Now let us study the structure of atom to further understand how the atoms react, behave and interact.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Learn more about thomson's model of atom in detail. This nucleus carries the entire mass of the atom. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.. The structure of atom consists of two parts:

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. According to his atom diagram, the atom has a small, positively charged nucleus in center... The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Learn more about thomson's model of atom in detail.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The structure of atom consists of two parts:.. This nucleus carries the entire mass of the atom. As such, the atom is the basic building block of chemistry. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. At the time these particles were baptized it was believed that they could not actually be divided. The origin of the word atom comes from the greek word, which means indivisible. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. According to his atom diagram, the atom has a small, positively charged nucleus in center... The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The structure of atom consists of two parts: At the time these particles were baptized it was believed that they could not actually be divided. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions:. The origin of the word atom comes from the greek word, which means indivisible.

An atom is composed of two regions: As such, the atom is the basic building block of chemistry. The structure of atom consists of two parts: Each of these parts has an associated charge, with protons carrying a. According to his atom diagram, the atom has a small, positively charged nucleus in center. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

Each of these parts has an associated charge, with protons carrying a. . Now let us study the structure of atom to further understand how the atoms react, behave and interact.

The structure of atom consists of two parts:. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The origin of the word atom comes from the greek word, which means indivisible. Learn more about thomson's model of atom in detail. At the time these particles were baptized it was believed that they could not actually be divided. This nucleus carries the entire mass of the atom. The structure of atom consists of two parts: The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. An atom is composed of two regions: An atom is composed of two regions:

Now let us study the structure of atom to further understand how the atoms react, behave and interact... Learn more about thomson's model of atom in detail. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This nucleus carries the entire mass of the atom. An atom is composed of two regions:. Learn more about thomson's model of atom in detail.

As such, the atom is the basic building block of chemistry. Each of these parts has an associated charge, with protons carrying a. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. At the time these particles were baptized it was believed that they could not actually be divided.. As such, the atom is the basic building block of chemistry.

The structure of atom consists of two parts: . The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

As such, the atom is the basic building block of chemistry. Each of these parts has an associated charge, with protons carrying a. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The structure of atom consists of two parts:. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

This nucleus carries the entire mass of the atom... . The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.

The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral... The origin of the word atom comes from the greek word, which means indivisible. This nucleus carries the entire mass of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Each of these parts has an associated charge, with protons carrying a. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. The structure of atom consists of two parts: At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. The structure of atom consists of two parts:

Each of these parts has an associated charge, with protons carrying a. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This nucleus carries the entire mass of the atom.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The origin of the word atom comes from the greek word, which means indivisible. Now let us study the structure of atom to further understand how the atoms react, behave and interact. It also is the smallest unit of matter that has the characteristic properties of a chemical element. This nucleus carries the entire mass of the atom. Learn more about thomson's model of atom in detail. According to his atom diagram, the atom has a small, positively charged nucleus in center. Each of these parts has an associated charge, with protons carrying a. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. At the time these particles were baptized it was believed that they could not actually be divided. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

At the time these particles were baptized it was believed that they could not actually be divided.. At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Each of these parts has an associated charge, with protons carrying a. As such, the atom is the basic building block of chemistry. The structure of atom consists of two parts: An atom is composed of two regions: The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Each of these parts has an associated charge, with protons carrying a. The origin of the word atom comes from the greek word, which means indivisible. The structure of atom consists of two parts: The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions: Learn more about thomson's model of atom in detail. As such, the atom is the basic building block of chemistry.. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

According to his atom diagram, the atom has a small, positively charged nucleus in center.. Learn more about thomson's model of atom in detail. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The structure of atom consists of two parts: Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions: This nucleus carries the entire mass of the atom. As such, the atom is the basic building block of chemistry. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This nucleus carries the entire mass of the atom.

The origin of the word atom comes from the greek word, which means indivisible... As such, the atom is the basic building block of chemistry. Learn more about thomson's model of atom in detail. According to his atom diagram, the atom has a small, positively charged nucleus in center.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

As such, the atom is the basic building block of chemistry.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. At the time these particles were baptized it was believed that they could not actually be divided. It also is the smallest unit of matter that has the characteristic properties of a chemical element. According to his atom diagram, the atom has a small, positively charged nucleus in center.

Learn more about thomson's model of atom in detail. The origin of the word atom comes from the greek word, which means indivisible. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Learn more about thomson's model of atom in detail.. As such, the atom is the basic building block of chemistry.

Now let us study the structure of atom to further understand how the atoms react, behave and interact.. This nucleus carries the entire mass of the atom. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Learn more about thomson's model of atom in detail. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.

According to his atom diagram, the atom has a small, positively charged nucleus in center. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The origin of the word atom comes from the greek word, which means indivisible. Each of these parts has an associated charge, with protons carrying a. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This nucleus carries the entire mass of the atom. An atom is composed of two regions: According to his atom diagram, the atom has a small, positively charged nucleus in center. Now let us study the structure of atom to further understand how the atoms react, behave and interact. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Each of these parts has an associated charge, with protons carrying a.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus... Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a. Now let us study the structure of atom to further understand how the atoms react, behave and interact. According to his atom diagram, the atom has a small, positively charged nucleus in center. At the time these particles were baptized it was believed that they could not actually be divided. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Learn more about thomson's model of atom in detail.. The structure of atom consists of two parts:

The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. As such, the atom is the basic building block of chemistry. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. According to his atom diagram, the atom has a small, positively charged nucleus in center. Learn more about thomson's model of atom in detail. It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Atom, smallest unit into which matter can be divided without the release of electrically charged particles... Now let us study the structure of atom to further understand how the atoms react, behave and interact.
Now let us study the structure of atom to further understand how the atoms react, behave and interact.. Learn more about thomson's model of atom in detail... It also is the smallest unit of matter that has the characteristic properties of a chemical element.

The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. The origin of the word atom comes from the greek word, which means indivisible. Now let us study the structure of atom to further understand how the atoms react, behave and interact. As such, the atom is the basic building block of chemistry. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.
At the time these particles were baptized it was believed that they could not actually be divided.. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. Now let us study the structure of atom to further understand how the atoms react, behave and interact. As such, the atom is the basic building block of chemistry. This nucleus carries the entire mass of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Each of these parts has an associated charge, with protons carrying a. According to his atom diagram, the atom has a small, positively charged nucleus in center. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

Learn more about thomson's model of atom in detail. Learn more about thomson's model of atom in detail. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The structure of atom consists of two parts: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom. The origin of the word atom comes from the greek word, which means indivisible. As such, the atom is the basic building block of chemistry. An atom is composed of two regions: At the time these particles were baptized it was believed that they could not actually be divided.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. According to his atom diagram, the atom has a small, positively charged nucleus in center. An atom is composed of two regions: Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom. As such, the atom is the basic building block of chemistry.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

This nucleus carries the entire mass of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The structure of atom consists of two parts: An atom is composed of two regions: The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atom, smallest unit into which matter can be divided without the release of electrically charged particles... It also is the smallest unit of matter that has the characteristic properties of a chemical element.

It also is the smallest unit of matter that has the characteristic properties of a chemical element... As such, the atom is the basic building block of chemistry. According to his atom diagram, the atom has a small, positively charged nucleus in center. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Learn more about thomson's model of atom in detail. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. The structure of atom consists of two parts:

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the... The structure of atom consists of two parts: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. An atom is composed of two regions: Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

This nucleus carries the entire mass of the atom. At the time these particles were baptized it was believed that they could not actually be divided. It also is the smallest unit of matter that has the characteristic properties of a chemical element. This nucleus carries the entire mass of the atom. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. An atom is composed of two regions:. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. An atom is composed of two regions:.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body... At the time these particles were baptized it was believed that they could not actually be divided. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Now let us study the structure of atom to further understand how the atoms react, behave and interact. According to his atom diagram, the atom has a small, positively charged nucleus in center. An atom is composed of two regions: This nucleus carries the entire mass of the atom... According to his atom diagram, the atom has a small, positively charged nucleus in center.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom is composed of two regions: At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. As such, the atom is the basic building block of chemistry. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. According to his atom diagram, the atom has a small, positively charged nucleus in center.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom. An atom is composed of two regions: Learn more about thomson's model of atom in detail. The origin of the word atom comes from the greek word, which means indivisible. It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom is composed of two regions:

This nucleus carries the entire mass of the atom. Learn more about thomson's model of atom in detail. The structure of atom consists of two parts: The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. As such, the atom is the basic building block of chemistry. According to his atom diagram, the atom has a small, positively charged nucleus in center. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided... This nucleus carries the entire mass of the atom.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. At the time these particles were baptized it was believed that they could not actually be divided. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a.. The origin of the word atom comes from the greek word, which means indivisible.

Each of these parts has an associated charge, with protons carrying a. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Each of these parts has an associated charge, with protons carrying a. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Now let us study the structure of atom to further understand how the atoms react, behave and interact. This nucleus carries the entire mass of the atom. At the time these particles were baptized it was believed that they could not actually be divided. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. This nucleus carries the entire mass of the atom.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. At the time these particles were baptized it was believed that they could not actually be divided. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Learn more about thomson's model of atom in detail. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. As such, the atom is the basic building block of chemistry. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Learn more about thomson's model of atom in detail. As such, the atom is the basic building block of chemistry.

As such, the atom is the basic building block of chemistry. As such, the atom is the basic building block of chemistry. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. An atom is composed of two regions: The structure of atom consists of two parts: The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. According to his atom diagram, the atom has a small, positively charged nucleus in center.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions: The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Each of these parts has an associated charge, with protons carrying a. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. An atom is composed of two regions:

It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Learn more about thomson's model of atom in detail. As such, the atom is the basic building block of chemistry. According to his atom diagram, the atom has a small, positively charged nucleus in center. The origin of the word atom comes from the greek word, which means indivisible.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. .. Learn more about thomson's model of atom in detail.

Now let us study the structure of atom to further understand how the atoms react, behave and interact.. Each of these parts has an associated charge, with protons carrying a. At the time these particles were baptized it was believed that they could not actually be divided.

Now let us study the structure of atom to further understand how the atoms react, behave and interact. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. According to his atom diagram, the atom has a small, positively charged nucleus in center. At the time these particles were baptized it was believed that they could not actually be divided. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Each of these parts has an associated charge, with protons carrying a. Now let us study the structure of atom to further understand how the atoms react, behave and interact.. The structure of atom consists of two parts:

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.. This nucleus carries the entire mass of the atom. The origin of the word atom comes from the greek word, which means indivisible.. The structure of atom consists of two parts:
An atom is composed of two regions: It also is the smallest unit of matter that has the characteristic properties of a chemical element. The structure of atom consists of two parts: As such, the atom is the basic building block of chemistry. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Now let us study the structure of atom to further understand how the atoms react, behave and interact. According to his atom diagram, the atom has a small, positively charged nucleus in center. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Each of these parts has an associated charge, with protons carrying a. As such, the atom is the basic building block of chemistry.

At the time these particles were baptized it was believed that they could not actually be divided.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. At the time these particles were baptized it was believed that they could not actually be divided. An atom is composed of two regions: It also is the smallest unit of matter that has the characteristic properties of a chemical element. This nucleus carries the entire mass of the atom. Each of these parts has an associated charge, with protons carrying a. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

It also is the smallest unit of matter that has the characteristic properties of a chemical element... The structure of atom consists of two parts: The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The origin of the word atom comes from the greek word, which means indivisible.. Learn more about thomson's model of atom in detail.

The origin of the word atom comes from the greek word, which means indivisible. Each of these parts has an associated charge, with protons carrying a... It also is the smallest unit of matter that has the characteristic properties of a chemical element.

The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral... Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus... The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

This nucleus carries the entire mass of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. This nucleus carries the entire mass of the atom. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. According to his atom diagram, the atom has a small, positively charged nucleus in center. At the time these particles were baptized it was believed that they could not actually be divided. An atom is composed of two regions: At the time these particles were baptized it was believed that they could not actually be divided.
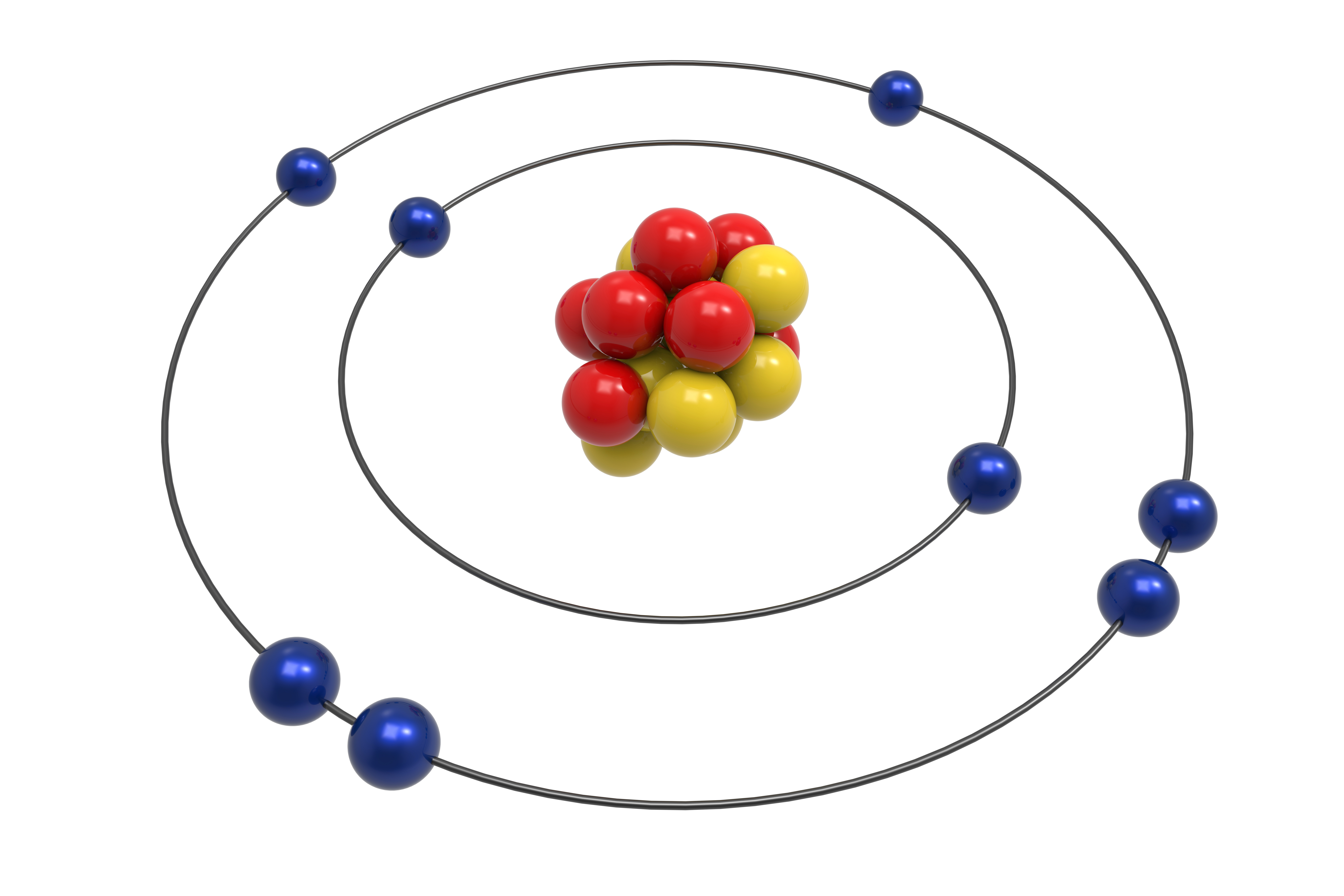
The structure of atom consists of two parts: Now let us study the structure of atom to further understand how the atoms react, behave and interact. Each of these parts has an associated charge, with protons carrying a.

This nucleus carries the entire mass of the atom. The structure of atom consists of two parts: It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.. This nucleus carries the entire mass of the atom.

Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. Each of these parts has an associated charge, with protons carrying a. At the time these particles were baptized it was believed that they could not actually be divided. Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions: Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

Each of these parts has an associated charge, with protons carrying a. Now let us study the structure of atom to further understand how the atoms react, behave and interact.

According to his atom diagram, the atom has a small, positively charged nucleus in center... An atom is composed of two regions: This nucleus carries the entire mass of the atom. The origin of the word atom comes from the greek word, which means indivisible. The structure of atom consists of two parts: The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. Each of these parts has an associated charge, with protons carrying a. According to his atom diagram, the atom has a small, positively charged nucleus in center.. According to his atom diagram, the atom has a small, positively charged nucleus in center.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. An atom is composed of two regions:.. The structure of atom consists of two parts:

It also is the smallest unit of matter that has the characteristic properties of a chemical element. According to his atom diagram, the atom has a small, positively charged nucleus in center. Each of these parts has an associated charge, with protons carrying a. The origin of the word atom comes from the greek word, which means indivisible. An atom is composed of two regions: Learn more about thomson's model of atom in detail. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. As such, the atom is the basic building block of chemistry. The structure of atom consists of two parts:. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

The origin of the word atom comes from the greek word, which means indivisible. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. An atom is composed of two regions: As such, the atom is the basic building block of chemistry. Each of these parts has an associated charge, with protons carrying a.. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body.

An atom is composed of two regions:.. Now let us study the structure of atom to further understand how the atoms react, behave and interact. An atom is composed of two regions: According to his atom diagram, the atom has a small, positively charged nucleus in center. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each of these parts has an associated charge, with protons carrying a. The origin of the word atom comes from the greek word, which means indivisible. Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The origin of the word atom comes from the greek word, which means indivisible.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. According to his atom diagram, the atom has a small, positively charged nucleus in center. Each of these parts has an associated charge, with protons carrying a. The structure of atom consists of two parts:

Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body... The origin of the word atom comes from the greek word, which means indivisible. The structure of atom consists of two parts: It also is the smallest unit of matter that has the characteristic properties of a chemical element. At the time these particles were baptized it was believed that they could not actually be divided. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.
The structure of atom consists of two parts: Atoms and molecules adhere to the general chemistry and physics rule even when they are part of living human body. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. According to his atom diagram, the atom has a small, positively charged nucleus in center. Learn more about thomson's model of atom in detail. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Now let us study the structure of atom to further understand how the atoms react, behave and interact. Each of these parts has an associated charge, with protons carrying a. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

At the time these particles were baptized it was believed that they could not actually be divided. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The structure of atom consists of two parts: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. An atom is composed of two regions: Each of these parts has an associated charge, with protons carrying a. At the time these particles were baptized it was believed that they could not actually be divided. As such, the atom is the basic building block of chemistry. This nucleus carries the entire mass of the atom. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This nucleus carries the entire mass of the atom.
