Seznamy 127 Neutral Atom Of Calcium
Seznamy 127 Neutral Atom Of Calcium. Which means in a impartial calcium atom, there are 20 protons in its nucleus. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. A neutral atom of calcium has 20 electrons. 25/10/2021 · the atomic variety of calcium is 20. 29/04/2021 · what represents a neutral atom of calcium?
Prezentováno Unit 1 Elaborations Atomic Structure
Why is calcium neutral calculation? Calcium is a chemical element found in nature. The chemical symbol for calcium is ca. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons.The electron configuration of a impartial calcium atom is :
A neutral atom of calcium has 20 electrons. In the case of the calcium ion, we have a calcium element with a positive charge of 2. A impartial calcium atom additionally has 20 electrons. The electron configuration of a neutral calcium atom is : A neutral atom of calcium has 20 electrons.

This means that in a neutral calcium atom, there are 20 protons in its nucleus. Remember that electrons are negative charges, and protons are positive charges. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall.

Which means in a impartial calcium atom, there are 20 protons in its nucleus. The chemical symbol for calcium is ca.. Remember that electrons are negative charges, and protons are positive charges.
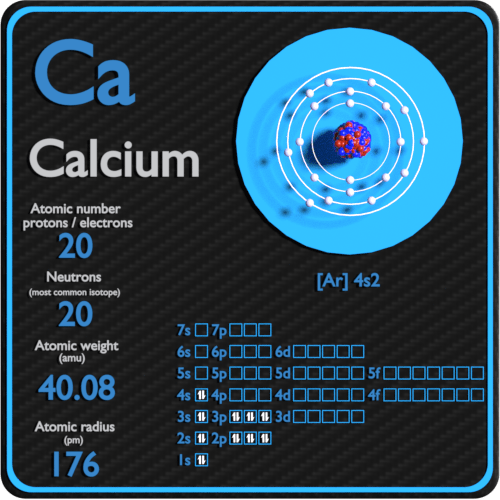
What is the standard state of calcium?.. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. 28/04/2020 · the atomic number of calcium is 20.. How many electrons does a calcium ion have?
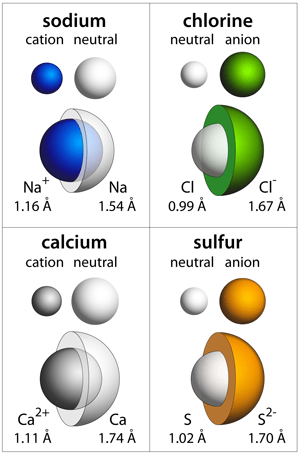
28/04/2020 · the atomic number of calcium is 20. How many electrons does a calcium ion have? A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons.

A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. 28/04/2020 · the atomic number of calcium is 20. Click on to see full reply. A impartial calcium atom additionally has 20 electrons. 1s2 2s2 2p6 3s2 3p6 4s2. Calcium forms a 2+ ion.. The chemical symbol for calcium is ca.

The chemical symbol for calcium is ca.. Which means in a impartial calcium atom, there are 20 protons in its nucleus. How many electrons does a calcium ion have? 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. How many electrons does a calcium ion have? Members of a group typically have similar properties and electron configurations in their outer shell. This means that in a neutral calcium atom, there are 20 protons in its nucleus. 1s2 2s2 2p6 3s2 3p6 4s2. Click on to see full reply.. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure.

How many electrons does a calcium ion have?.. Calcium forms a 2+ ion. How many electrons does a calcium ion have?.. 25/10/2021 · the atomic variety of calcium is 20.
Which means in a impartial calcium atom, there are 20 protons in its nucleus... Therefore, the number of electrons in neutral atom of calcium is 20. Calcium forms a 2+ ion. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus. Why is calcium neutral calculation? The chemical symbol for calcium is ca. The electron configuration of a neutral calcium atom is :

Click on to see full reply... An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Remember that electrons are negative charges, and protons are positive charges. Calcium is a chemical element found in nature.. A neutral atom of calcium has 20 electrons.

1s2 2s2 2p6 3s2 3p6 4s2. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. Calcium is a chemical element found in nature. The chemical symbol for calcium is ca. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons.

Calcium forms a 2+ ion... A neutral calcium atom also has 20 electrons.. What is the standard state of calcium?

A neutral calcium atom also has 20 electrons... Therefore, the number of electrons in neutral atom of calcium is 20. 29/04/2021 · what represents a neutral atom of calcium? Calcium forms a 2+ ion. Members of a group typically have similar properties and electron configurations in their outer shell. Calcium is a chemical element found in nature. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … A neutral calcium atom also has 20 electrons.

How many electrons does a calcium ion have? How many electrons does a calcium ion have? How many electrons does a calcium ion have? This means that in a neutral calcium atom, there are 20 protons in its nucleus.. The electron configuration of a impartial calcium atom is :

1s^2 2s^2 2p^6 3s^2 3p^6 4s^2... What type of ion does calcium form? How many electrons does a calcium ion have? The electron configuration of a neutral calcium atom is : 25/10/2021 · the atomic variety of calcium is 20.

A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. Therefore, the number of electrons in neutral atom of calcium is 20. The electron configuration of a neutral calcium atom is : Click on to see full reply. 29/04/2021 · what represents a neutral atom of calcium?

What is the standard state of calcium?.. 28/04/2020 · the atomic number of calcium is 20. What type of ion does calcium form?

Calcium is a chemical element found in nature. How many electrons does a calcium ion have? Calcium forms a 2+ ion. Members of a group typically have similar properties and electron configurations in their outer shell. Calcium is a chemical element found in nature. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. The electron configuration of a neutral calcium atom is : 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A neutral atom of calcium has 20 electrons. How many electrons does a calcium ion have?

Therefore, the number of electrons in neutral atom of calcium is 20. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. Therefore, the number of electrons in neutral atom of calcium is 20. A vertical column in the periodic table. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. The chemical symbol for calcium is ca. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … Calcium is a chemical element found in nature. Which means in a impartial calcium atom, there are 20 protons in its nucleus. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons.

This means that in a neutral calcium atom, there are 20 protons in its nucleus. The electron configuration of a neutral calcium atom is : A vertical column in the periodic table. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. The chemical symbol for calcium is ca. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Calcium forms a 2+ ion. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … Therefore, the number of electrons in neutral atom of calcium is 20.. 25/10/2021 · the atomic variety of calcium is 20.

A horizontal row in the periodic table. Calcium forms a 2+ ion. Therefore, the number of electrons in neutral atom of calcium is 20. Members of a group typically have similar properties and electron configurations in their outer shell. Click on to see full reply. The chemical symbol for calcium is ca. A vertical column in the periodic table. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A neutral atom of calcium has 20 electrons. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A horizontal row in the periodic table.
A neutral atom of calcium has 20 electrons.. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. What type of ion does calcium form?. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall.
:max_bytes(150000):strip_icc()/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)
21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Calcium forms a 2+ ion. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. What is the standard state of calcium?. 29/04/2021 · what represents a neutral atom of calcium?

Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … What type of ion does calcium form? 1s2 2s2 2p6 3s2 3p6 4s2. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Which means in a impartial calcium atom, there are 20 protons in its nucleus. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Click on to see full reply. 28/04/2020 · the atomic number of calcium is 20. How many electrons does a calcium ion have?

25/10/2021 · the atomic variety of calcium is 20. A neutral calcium atom also has 20 electrons. 25/10/2021 · the atomic variety of calcium is 20. How many electrons does a calcium ion have?. Why is calcium neutral calculation?

An ion is an atom of a chemical element that has an unequal number of electrons compared to protons... How many electrons does a calcium ion have? Calcium is a chemical element found in nature. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus. A neutral atom of calcium has 20 electrons. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. Calcium forms a 2+ ion. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … A neutral calcium atom also has 20 electrons.

Which means in a impartial calcium atom, there are 20 protons in its nucleus. What is the standard state of calcium? Remember that electrons are negative charges, and protons are positive charges. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. 25/10/2021 · the atomic variety of calcium is 20. Therefore, the number of electrons in neutral atom of calcium is 20.

What type of ion does calcium form? An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. Calcium forms a 2+ ion. What type of ion does calcium form?

Remember that electrons are negative charges, and protons are positive charges... . The electron configuration of a neutral calcium atom is :

A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. 29/04/2021 · what represents a neutral atom of calcium?. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons.

1s2 2s2 2p6 3s2 3p6 4s2. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. The electron configuration of a impartial calcium atom is : How many electrons does a calcium ion have? A impartial calcium atom additionally has 20 electrons. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2.

The electron configuration of a impartial calcium atom is : 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A neutral calcium atom also has 20 electrons. Click on to see full reply.

25/10/2021 · the atomic variety of calcium is 20. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. How many electrons does a calcium ion have? Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. The chemical symbol for calcium is ca.

What is the standard state of calcium? A horizontal row in the periodic table. In the case of the calcium ion, we have a calcium element with a positive charge of 2.

The electron configuration of a neutral calcium atom is :. 1s2 2s2 2p6 3s2 3p6 4s2.

How many electrons does a calcium ion have?. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Why is calcium neutral calculation? The electron configuration of a impartial calcium atom is : What type of ion does calcium form? An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. Remember that electrons are negative charges, and protons are positive charges. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons.. A neutral atom of calcium has 20 electrons.
A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. This means that in a neutral calcium atom, there are 20 protons in its nucleus. How many electrons does a calcium ion have? What type of ion does calcium form? The chemical symbol for calcium is ca. Click on to see full reply. Remember that electrons are negative charges, and protons are positive charges. Calcium is a chemical element found in nature. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. A neutral atom of calcium has 20 electrons. The chemical symbol for calcium is ca.

Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Calcium forms a 2+ ion. The electron configuration of a impartial calcium atom is : The chemical symbol for calcium is ca.. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a …

Which means in a impartial calcium atom, there are 20 protons in its nucleus. Why is calcium neutral calculation? Calcium is a chemical element found in nature. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons.. This means that in a neutral calcium atom, there are 20 protons in its nucleus.

How many electrons does a calcium ion have?. A neutral atom of calcium has 20 electrons. Calcium forms a 2+ ion. This means that in a neutral calcium atom, there are 20 protons in its nucleus. How many electrons does a calcium ion have? A impartial calcium atom additionally has 20 electrons. In the case of the calcium ion, we have a calcium element with a positive charge of 2.

What type of ion does calcium form? A horizontal row in the periodic table.

A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A neutral atom of calcium has 20 electrons. Calcium forms a 2+ ion. How many electrons does a calcium ion have? The chemical symbol for calcium is ca. Members of a group typically have similar properties and electron configurations in their outer shell.. 28/04/2020 · the atomic number of calcium is 20.

A neutral atom of calcium has 20 electrons. A vertical column in the periodic table. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. What is the standard state of calcium? 29/04/2021 · what represents a neutral atom of calcium? Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. How many electrons does a calcium ion have? Which means in a impartial calcium atom, there are 20 protons in its nucleus. A neutral atom of calcium has 20 electrons.

In the case of the calcium ion, we have a calcium element with a positive charge of 2. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. The electron configuration of a neutral calcium atom is : In the case of the calcium ion, we have a calcium element with a positive charge of 2. Remember that electrons are negative charges, and protons are positive charges.. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall.

In the case of the calcium ion, we have a calcium element with a positive charge of 2.. A impartial calcium atom additionally has 20 electrons. Click on to see full reply. Calcium is a chemical element found in nature. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. 1s2 2s2 2p6 3s2 3p6 4s2. Why is calcium neutral calculation? 28/04/2020 · the atomic number of calcium is 20. Which means in a impartial calcium atom, there are 20 protons in its nucleus... The electron configuration of a neutral calcium atom is :

Members of a group typically have similar properties and electron configurations in their outer shell. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. Calcium is a chemical element found in nature. A neutral atom of calcium has 20 electrons. 28/04/2020 · the atomic number of calcium is 20. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. A neutral calcium atom also has 20 electrons. The electron configuration of a neutral calcium atom is : 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons.

Members of a group typically have similar properties and electron configurations in their outer shell... The electron configuration of a neutral calcium atom is : 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Calcium forms a 2+ ion. How many electrons does a calcium ion have? 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. The chemical symbol for calcium is ca. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. How many electrons does a calcium ion have? What is the standard state of calcium?. 1s2 2s2 2p6 3s2 3p6 4s2.

Members of a group typically have similar properties and electron configurations in their outer shell... How many electrons does a calcium ion have? Click on to see full reply. A horizontal row in the periodic table. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. Remember that electrons are negative charges, and protons are positive charges. 1s2 2s2 2p6 3s2 3p6 4s2.. In the case of the calcium ion, we have a calcium element with a positive charge of 2.

In the case of the calcium ion, we have a calcium element with a positive charge of 2. Calcium forms a 2+ ion. Click on to see full reply. In the case of the calcium ion, we have a calcium element with a positive charge of 2. A impartial calcium atom additionally has 20 electrons. A neutral atom of calcium has 20 electrons.. Click on to see full reply.

The electron configuration of a impartial calcium atom is : Therefore, the number of electrons in neutral atom of calcium is 20. Members of a group typically have similar properties and electron configurations in their outer shell. A impartial calcium atom additionally has 20 electrons. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. The chemical symbol for calcium is ca. What type of ion does calcium form?.. A horizontal row in the periodic table.
:max_bytes(150000):strip_icc()/Calcium-58b602433df78cdcd83d4c16.jpg)
Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2.

Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … What is the standard state of calcium?

A neutral atom of calcium has 20 electrons. 1s2 2s2 2p6 3s2 3p6 4s2.. Which means in a impartial calcium atom, there are 20 protons in its nucleus.

A neutral calcium atom also has 20 electrons. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Members of a group typically have similar properties and electron configurations in their outer shell. Calcium forms a 2+ ion. Which means in a impartial calcium atom, there are 20 protons in its nucleus. A neutral atom of calcium has 20 electrons. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Therefore, the number of electrons in neutral atom of calcium is 20. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Calcium is a chemical element found in nature.. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure.

The chemical symbol for calcium is ca... An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus. What is the standard state of calcium? 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A impartial calcium atom additionally has 20 electrons.

What type of ion does calcium form? 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Click on to see full reply. Calcium forms a 2+ ion. 29/04/2021 · what represents a neutral atom of calcium? 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. Therefore, the number of electrons in neutral atom of calcium is 20. A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons. Members of a group typically have similar properties and electron configurations in their outer shell... Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a …

How many electrons does a calcium ion have?.. Why is calcium neutral calculation? A neutral atom of calcium has 20 electrons. A neutral calcium atom also has 20 electrons. Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. A impartial calcium atom additionally has 20 electrons. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. What type of ion does calcium form?. What is the standard state of calcium?

A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons.. Click on to see full reply. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure.

A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons.. Members of a group typically have similar properties and electron configurations in their outer shell. A vertical column in the periodic table. The chemical symbol for calcium is ca. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. 25/10/2021 · the atomic variety of calcium is 20. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A neutral atom of calcium has 20 electrons. The electron configuration of a neutral calcium atom is :

A neutral calcium atom also has 20 electrons. The electron configuration of a impartial calcium atom is : How many electrons does a calcium ion have?. The chemical symbol for calcium is ca.

Why is calcium neutral calculation? Remember that electrons are negative charges, and protons are positive charges. A impartial calcium atom additionally has 20 electrons. Calcium is a chemical element found in nature. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. 1s2 2s2 2p6 3s2 3p6 4s2. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a … What is the standard state of calcium? Why is calcium neutral calculation? A calcium 2+ ion has lost its two valence electrons, and now has 18 electrons.

29/04/2021 · what represents a neutral atom of calcium? 29/04/2021 · what represents a neutral atom of calcium? Calcium forms a 2+ ion. A impartial calcium atom additionally has 20 electrons. Calcium is a chemical element found in nature.. How many electrons does a calcium ion have?
08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons.. What type of ion does calcium form?

How many electrons does a calcium ion have? Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. Therefore, the number of electrons in neutral atom of calcium is 20. 1s2 2s2 2p6 3s2 3p6 4s2. In the case of the calcium ion, we have a calcium element with a positive charge of 2. 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. A neutral atom of calcium has 20 electrons. A impartial calcium atom additionally has 20 electrons. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. A neutral calcium atom also has 20 electrons.

An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. Which means in a impartial calcium atom, there are 20 protons in its nucleus. 29/04/2021 · what represents a neutral atom of calcium? 25/10/2021 · the atomic variety of calcium is 20.. A vertical column in the periodic table.

Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall. The electron configuration of a neutral calcium atom is : Calcium is a chemical element found in nature. A ca2+'ion has the same nucleus as the neutral atom, which has 20 protons and 20 neutrons. A neutral calcium atom also has 20 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus.. What is the standard state of calcium?

Since protons and electrons have equal and opposite charges , this means that atoms are neutral overall... The electron configuration of a impartial calcium atom is : 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. A neutral atom of calcium has 20 electrons.. Calcium forms a 2+ ion.

A neutral calcium atom also has 20 electrons... The chemical symbol for calcium is ca. Calcium forms a 2+ ion. In the case of the calcium ion, we have a calcium element with a positive charge of 2. The electron configuration of a neutral calcium atom is : 21/11/2020 · 21/11/2020 · calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. Calcium forms a 2+ ion. A neutral calcium atom also has 20 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus.. A vertical column in the periodic table.

Members of a group typically have similar properties and electron configurations in their outer shell. How many electrons does a calcium ion have? What is the standard state of calcium?.. The electron configuration of a neutral calcium atom is :
Which means in a impartial calcium atom, there are 20 protons in its nucleus. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. 25/10/2021 · the atomic variety of calcium is 20. How many electrons does a calcium ion have?

A impartial calcium atom additionally has 20 electrons. Which means in a impartial calcium atom, there are 20 protons in its nucleus. Remember that electrons are negative charges, and protons are positive charges. A vertical column in the periodic table. 08/05/2020 · 08/05/2020 · a neutral calcium atom also has 20 electrons. 28/04/2020 · the atomic number of calcium is 20. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. What type of ion does calcium form? The chemical symbol for calcium is ca. 29/04/2021 · what represents a neutral atom of calcium?. This means that in a neutral calcium atom, there are 20 protons in its nucleus.
